Modelling oxygen transfer within a dual chamber system for insulin-producing cell transportation under ambient conditions
Ruth Tarpey1,2, Daniel A Domingo-Lopez1,2, Benjamin Brennan1,2, Abhik Mallick1,2, Garry P Duffy1,2, Ruth E Levey1,2.
1Anatomy and Regenerative Medicine Institute (REMEDI), University of Galway, Galway, Ireland; 2CÚRAM Centre for Research in Medical Devices, University of Galway, Galway, Ireland
Introduction
Cryopreservation of islets and stem cell derived insulin-producing cells are often used to extend shelf life during transport1. This freeze-thaw process can affect cell viability, functionality2, and impair glucose-mediated insulin secretion3,4. Transporting cell products under ambient conditions could offer multiple benefits including reduction of cold stress induced cell damage, negate the need for cold chain storage2 and streamline the supply chain. However, ensuring oxygenation is a critical factor for survival and function of viable cells5. In this study, we describe an oxygenated transport system, capable of facilitating ambient cell shipments. Additionally, a computational framework to investigate oxygen transfer within the system and to determine the longevity of cells in transit is be detailed.
Methods
The dual chamber device as shown in Figure 1a consists of two chambers separated by a gas permeable membrane. One chamber is filled with an oxygenated perfluorodecalin (PFD) anao-emulsion (blue) that supplies oxygen to cells suspended in hyaluronic acid (green) as it diffuses across an oxygen concentration gradient.
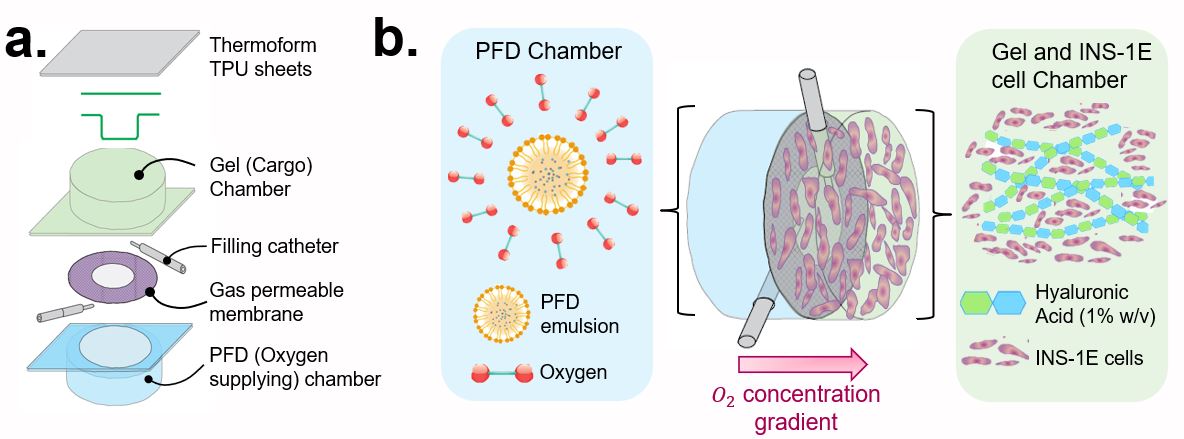
The use of this dual chamber device as a potential solution for international ambient cell transportation was assessed, where the devices were filled with insulin secreting rat insulinoma (INS-1E) cells and sent under ambient temperature conditions (Figure 2a). The live cells showed normal functionality in Pseudoislet formation after 48 hrs at ambient temperature (Figure 2c) when compared with those poorly formed by Out of Cryo Thaw cells (Figure 2b).

To understand the oxygen transfer within the system and assess the longevity of the cells in transit, oxygen sensors are placed within the chambers to capture the movement across the gradient, and their respective initial oxygen concentrations. Additionally, the diffusivity of the device materials was calculated using a steady-state membrane diffusion set-up, while the diffusivity of the gel and PFD were determined assuming equimolar counter diffusion into an infinite plane4. The dual chamber system is simulated using COMSOL Multiphysics transport of dilute species. A 2D model of the domains described above is created (Figure 3a). The oxygen consumption of the INS-1E cells is incorporated using Michaelis-Menten kinetics, combining the experimental oxygen consumption rate normalised per INS-1E cell and the density of cells in the device4.
Results
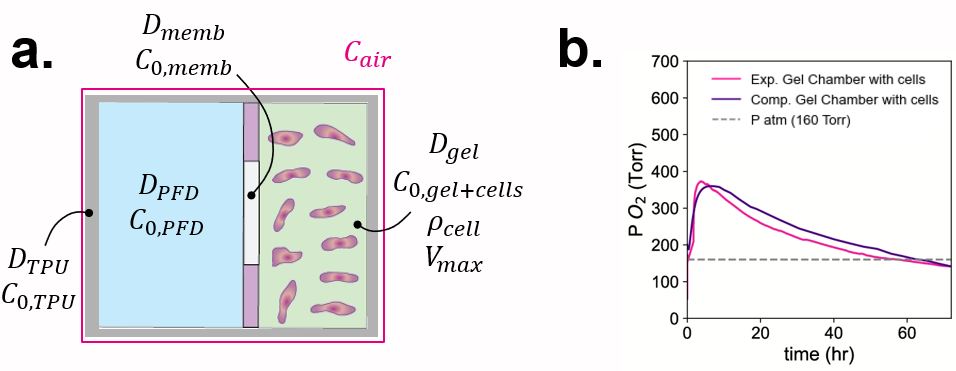
When comparing the experimental and predicted computational oxygen partial pressure within the INS-1E cell chamber, the model captures the movement of oxygen within the system, with the same oxygen peak and decrease resembling an absorption and elimination pharmacokinetic model. Consequently, the Michaelis-Menten reaction could mimic the oxygen consumption of the cells in ambient transit.
Conclusion
The accurate fit between simulated and experimental results, allows this computational framework to be used as a tool to correlate cell quality attributes as indicators of viability to the in-silico results, and subsequently guide device design and cell loading parameters to extend the oxygen durability of cells within the system and the corresponding ambient shipment duration.
CÚRAM is co-funded by the Science Foundation Ireland under Ireland’s European Structural and Investment Fund Grant Number 13/RC/2073_P2.
[1] Shah N. 2020. Autologous CAR T-cell therapies supply chain: challenges and opportunities? Cancer Gene Ther. 2020; 27: 799–809
[2] Hedayati M. Recent developments in cell shipping methods. Biotechnol. Bioeng. 2022; 119: 2985–3006.
[3] Shimada M. The Fragility of Cryopreserved Insulin-producing Cells Differentiated from Adipose-tissue-derived Stem Cells. Cell Transplantation. 2020; 29: 0963689720954798
[4] Rajotte R.V. 2001. Novel approaches to cryopreservation of human pancreatic islets. Transplantation. 2001; 72: 1005–1011.
[5] Colton C.K. Oxygen consumption and diffusion in assemblages of respiring spheres: Performance enhancement of a bioartificial pancreas. Chem. Eng. Sci. 2009; 64: 4470–4487