Novel 3D Cell Culture Technique Utilizing a Silicon Titanium Diboride Micropatterned Substrate for Differentiating Mesenchymal Stem Cells into Insulin Producing Cells
Jefferson Friguglietti1, Maram Quttina1, Wanda Wosik1, A. Osama Gaber2, Omaima Sabek2, Fatima Merchant1.
1Engineering Technology, University of Houston, Houston, TX, United States; 2Department of Surgery, Houston Methodist Hospital Research Institute, Houston, TX, United States
Introduction: 3D cell culture techniques are increasingly used in stem cell tissue engineering since they better mimic the in vivo environments compared to conventional 2D culture. Current 3D culture methods include less complex suspension methods such as hanging well and ultra-low attachment plate, to more advance methods incorporating scaffold designs to support cell-cell and cell-extracellular matrix interaction. In this study we investigate a novel microfabricated silicon-titanium diboride (Si-TiB2) substrate’s efficacy in increasing function of differentiated mesenchymal stem cells (MSCs) into insulin producing cells (IPCs). This substrate provides a 3D microenvironment (aggregates) and geometric (pattern shape), mechanical (stiffness gradients) and biochemical (selectively adsorbed proteins) cues that are critical for cell differentiation [1].
Methods: Utilizing photolithography technique, e-beam deposited TiB2 layers were fabricated on Si, wherein differences in their surface properties (hardness, stiffness, wetness, and electrical charge), enable selective adsorption of specific proteins on the micropatterns. Human MSCs from adult bone marrow were cultured on the substrate, and following a seven-day culture period subjected to a differentiation protocol [2]. Analyses included assessments of morphology, biomarkers and biomechanical properties of aggregates before differentiation. Functional analysis included quantification of c-peptide following a glucose stimulated insulin response (GSIR) assay,and qPCR analysis of key biomarkers.
Results: Morphological analysis of the cytoskeleton through immunofluorescence staining (f-actin, green) revealed increased rounded morphology of cells within the multicellular aggregates confirming a 3D culture environment on the Si-TiB2 substrate. Additionally, immunofluorescence staining for biomarkers (n-cadherin, red) showed increased expression within the rounded core indicating cell-cell interaction with the aggregates. Further BioAFM analysis indicated cells with higher elastic modulus in the center of aggregates compared to cells on the edges of the micropatterns (p < 0.05). GSIR assay showed MSCs differentiated on the Si-TiB2 substrate had a better response to high glucose stimulation when compared to those differentiated in 2D monolayers in tissue culture plates (p < 0.05). qPCR analysis showed significant differences on IPCs differentiated on the substrate when compared to those differentiated using 3D culture approach of low-attachment plates.
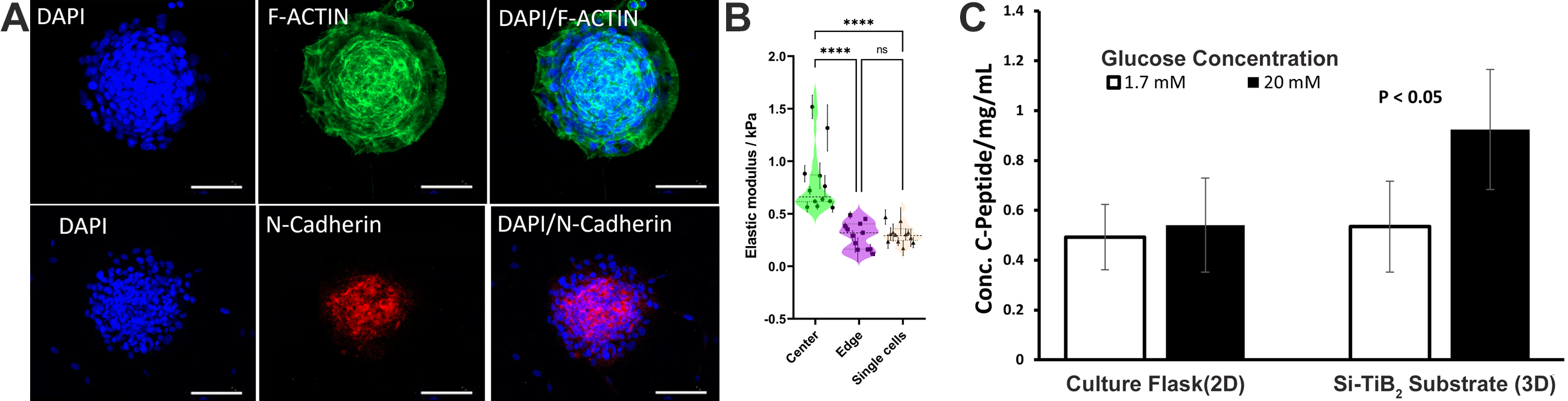
Conclusion: In this study the Si-TiB2 substrate, promoted the controlled (size and number) formation of aggregates providing a 3D microenvironment. MSCs differentiated in 3D culture responded significantly better to high glucose stimulation. This warrants future work evaluating other published chemical differentiation regimes to improve function of differentiated IPCs.
Supported in part by funding from the University of Houston High Priority Research Seed Grant and the University of Houston - Baylor College of Medicine Collaborative Seed Funding.
[1] J. Friguglietti, et. al, “Novel Silicon Titanium Diboride Micropatterned Substrates for Cellular Patterning,” Biomaterials. 2020; 244.
[2] M. M. Gabr, et. al, “Insulin-producing cells from adult human bone marrow mesenchymal stem cells control streptozotocin-induced diabetes in nude mice,” Cell Transplant. 2013;22(1):133-145.