
On-chip maturation and characterization of iPSC-derived Langerhanoids
Mahira Mehanović1, Emily Tubbs1, Frederique Kermarrec1, Karine Raymond1,2, Fabrice Navarro3, Delphine Freida1, Xavier Gidrol1.
1Biomics,Inserm, IRIG,CEA, University Grenoble Alpes, Grenoble, France; 2Department of Anatomy and Embryology, Leiden University Medical Center, Leiden, Netherlands; 3DTBS, LETI, CEA, University Grenoble Alpes, Grenoble, France
Type 1 diabetes (T1D) results from immune-mediated β cells destruction in islets of Langerhans leading to absolute insulin deficiency. Stem cells (SCs) represent potentially unlimited source of new insulin producing β cells for T1D cell replacement therapy and disease modeling. The SC-derived β cells have been successfully obtained in vitro through stepwise differentiation process, but improvements in their maturity are still needed. In regards, advances in culture systems such as microfluidic perfusion systems could enhance SC-derived islet functionality by providing biomimetic microenvironment in dynamic conditions. Here, we present generation of induced pluripotent stem cell (iPSC) derived islet organoids (i-Langerhanoids) using 2D and 3D models and microfluidic perfusion model that enables functional studies on one single i-Langerhanoids.
The iPSCs were differentiated into pancreatic endoderm in Matrigel-coated plates following step-wise protocol (1). Further differentiation into β cells was carried in 3D microwells culture that enabled enrichment of pancreatic progenitors and formation of uniformly sized aggregates. At the end of differentiation, qPCR was performed to access expression of β cell-specific markers. The glucose-stimulated insulin secretion and total insulin content were measured with ELISA. The microfluidic serpentine-shaped channel was designed with U-cup region dedicated to trapping single islets with diameter of 200-400 μm, as described previously (2).
The i-Langerhanoids obtained were positive for β cell-specific markers, but insulin secretion levels were lower compared to human cadaveric islets. Transfer of pancreatic progenitors to 3D microwells resulted in high yield of uniformly sized organoids. To further characterize and improve β cell response to glucose, i-Langerhanoids were trapped in microfluidic perfusion chip using hydrodynamic resistance at the flow rate of Q = 100 μL/min.
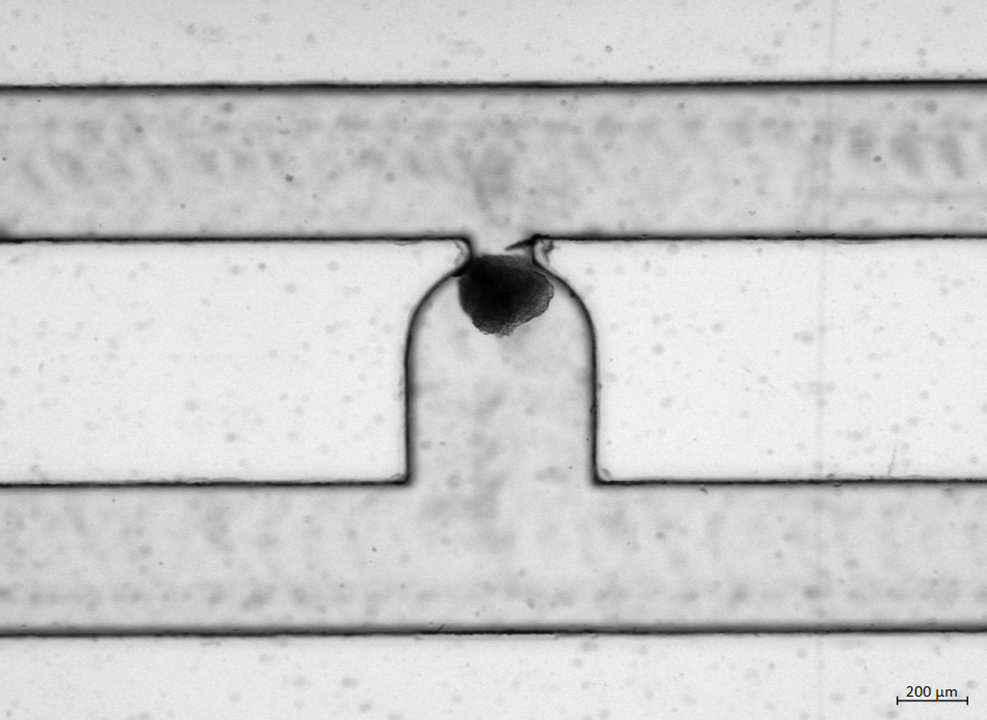
Here we have shown successful automated entrapment of i-Langerhanoids on the chip where the effect of static and dynamic culture will be studied. Next steps include integration of functional vascularized organoids-on-chip by adding endothelial cells (3). This will provide beneficial cues for islet growth, structure, and function, but also enable long-term culture. Improvements of physiological function will be monitored by glucose stimulated insulin secretion assay on-chip. Overall, combination of different cell culture systems and inclusion of heterogeneous cell types could bring SC-derived β cells closer to native pancreatic islets and provide model to study interactions underlying T1D.
[1] Fantuzzi F. et al. Frontiers in cell and developmental biology 2022;10:967765
[2] Quintard C. et al. Biosensors & bioelectronics 2022;202:113967
[3] Quintard C. et al. bioRxiv 2021;12.29.474327
Video Posters - Log in to view