
Nondestructive, longitudinal, 3D viability and functionality assessment in a multi-well plate system using EPR oxygen imaging
Mrignayani Kotecha1.
1Oxygen Measurement Core, O2M Technologies, LLC, Chicago, IL, United States
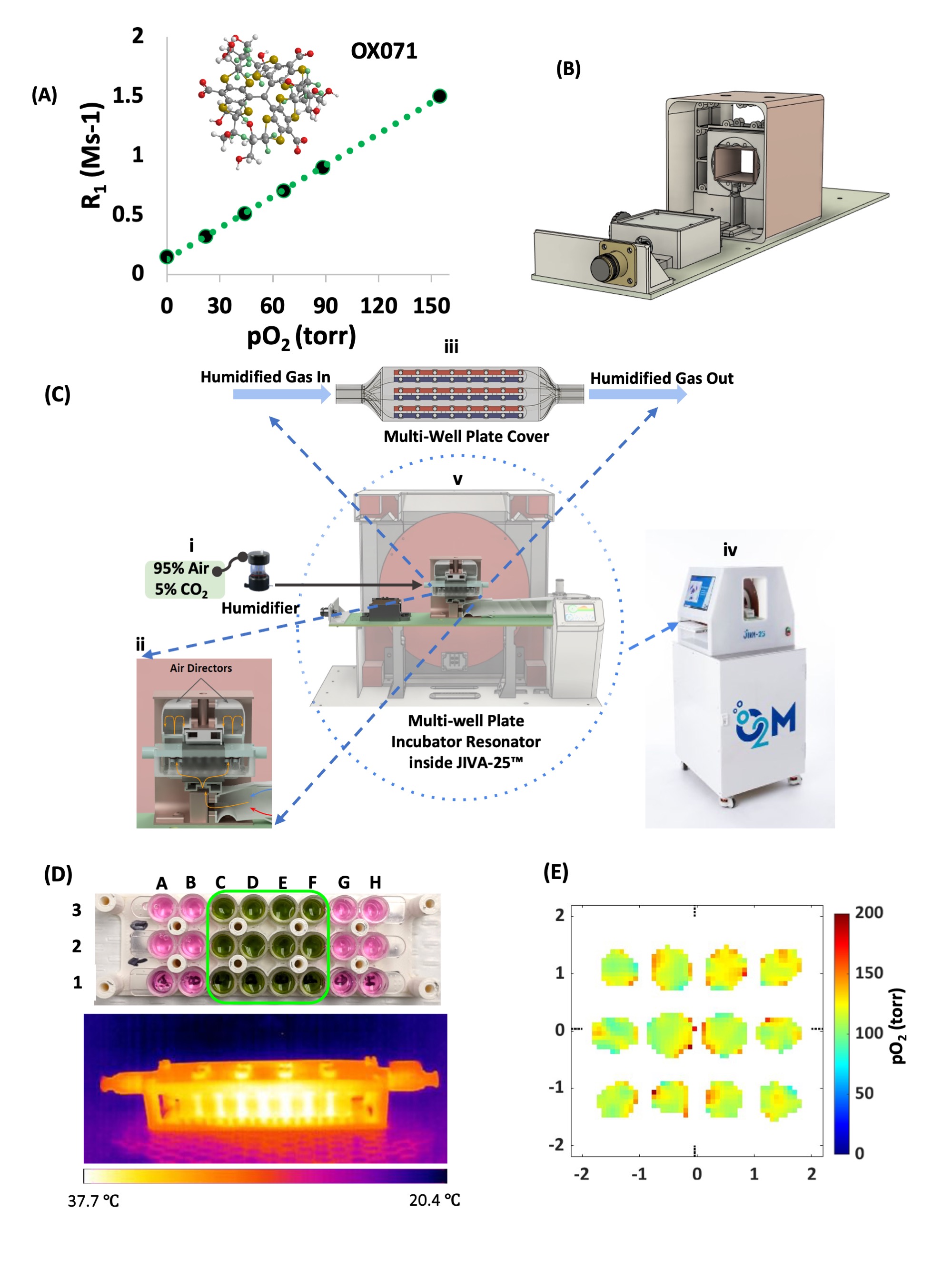
Oxygen consumption rate (OCR) has been shown to correlate positively with islet functionality. However, methods to measure OCR are destructive and are inadequate for cell plus scaffold system commonly used for islet transplantation devices. Here, we report the noninvasive cell viability assessment using trityl OX071-based electron paramagnetic resonance oxygen imaging (EPROI) in a multi-well plate. Recently, O2M introduced the first preclinical EPROI instrument named JIVA-25™, which is a 25 mT instrument operating at 720 MHz radiofrequency.
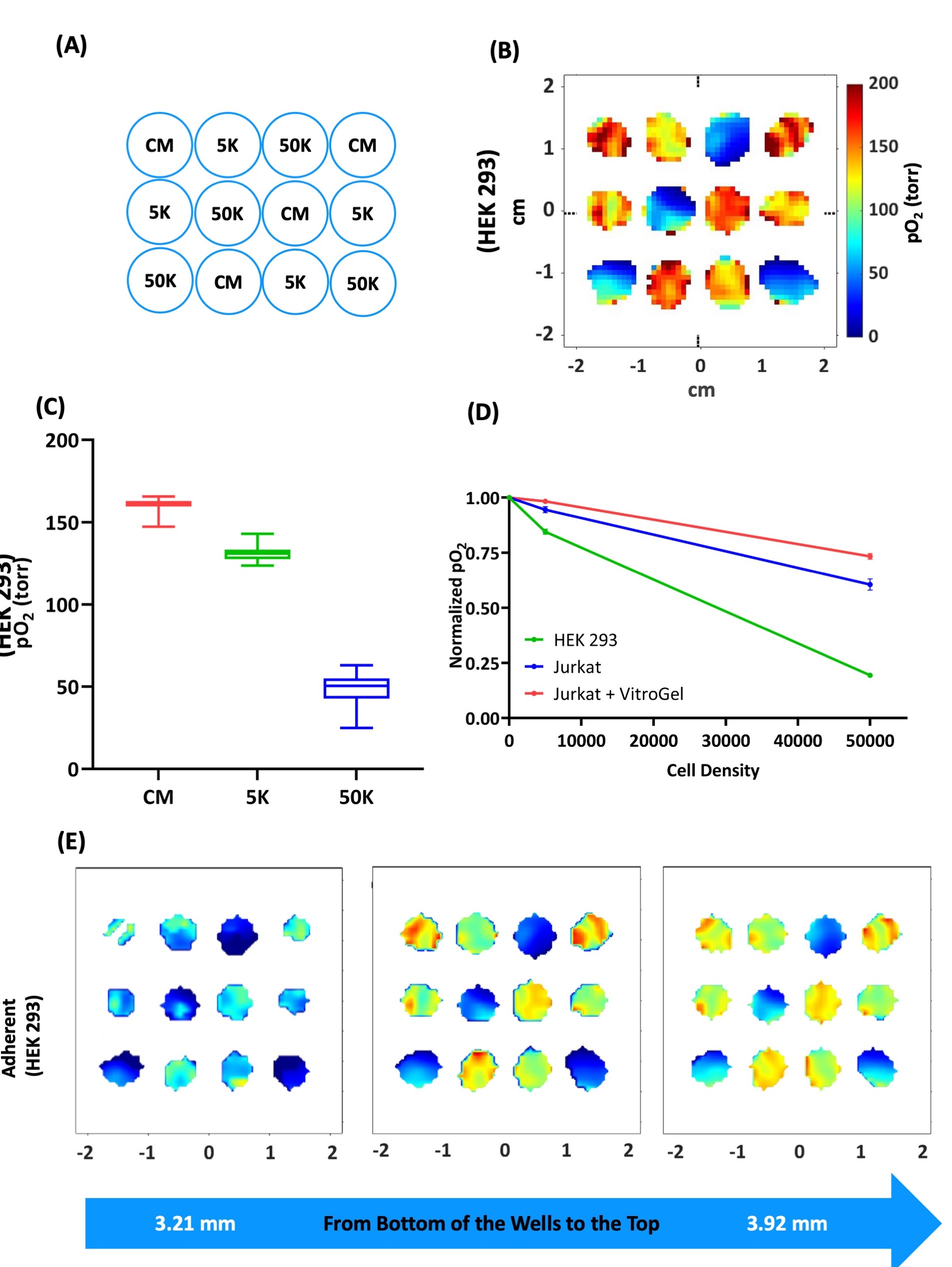
We developed a novel multi-well plate incubator-resonator (MWIR) platform (Figure 1) in conjunction with JIVA-25™ to perform pO2 imaging of live cells in 96-well plates during planar 2D culture and during culture in a 3D hydrogel scaffold. The MWIR performs the pO2 imaging in the middle 12 wells of 3 stripwells while maintaining the temp. at 37 °C and the constant flow of humidified gas mixture (f.e. 95% air & 5% CO2) to maintain incubator-like environment. The pO2 maps of cells allow the assessment of viable functional cells without destroying or sectioning them in the process. The MWIR system allows the longitudinal pO2 imaging up to 24 hours while keeping the cells viable. We performed the proof-of-concept cell viability measurements for an adherent HEK 293 cells, non-adherent Jurkat cells, and Jurkat cells in VitroGel (Figure 2).
This work demonstrates the noninvasive cell viability assessment in a multi-well plate using EPR oxygen imaging. We show that three-dimensional pO2 maps are indicative of functional and viable cells. We show that the functional/viable cells can be visualized using oxygen maps longitudinally without destroying them in the process. Using an adherent cell line (HEK-293) and a non-adherent cell line (Jurkat), we demonstrate the importance of three-dimensional cell viability assessment (Figure 2). We demonstrate the feasibility of cell viability assessment nondestructively in a cell-scaffold system comprising Jurkat cells and VitroGel, thus opening a new era of cell viability measurements in artificial tissue grafts and cell replacement devices. This work can be extended to perform real time OCR measurements for native and stem-cell derived islets in media or in biomaterials and under various oxygen conditions. Such measurements can be used to optimize the cell seeding density, biomaterial properties, and choosing the optimum device size for efficient beta cell replacement devices.
NSF grant 2028829 to O2M (PI: Kotecha).
[1] 1. Cell Viability Apparatus, System, and Methods Thereof, US patent application serial no. 63425187; Inventors: Mrignayani Kotecha, Boris Epel, Eliyas Siddiqui, Safa Hameed; Assignee: O2M Technologies, LLC; Filing date: Nov 14th, 2022.
[2] 2. Animal Temperature Control Apparatus and Methods Thereof, PCT international patent application, serial no. PCT/US2022/079482; Inventors: Mrignayani Kotecha, Boris Epel, Marcelo Gutierrez-Miranda; Assignee: O2M Technologies, LLC; Filing date: Nov 8th, 2022, priority date: Nov 9th, 2021.