
Toward a full-scale vascularized and immediately perfused beta cell graft for surgical implantation
Daniel T Bowers1, Manjot Singh1, Andrew Ford1, Daniel Cheng1, Susan Nelson1, Nick Colucci1, Thomas Gallegos1, Charles Klassen1, Harald C Ott1,2.
1IVIVA Medical, Woburn, MA, United States; 2Center for Organ Engineering, MGH, Boston, MA, United States
Introduction:
Native pancreatic islets receive blood flow of 10-30ml/min which is important for physiological response to glucose fluctuations. However, most current stem cell derived beta-cell culture and encapsulation technologies do not include perfusion. The fact that stem cell derived beta-cells require up to months to mature in vivo further underlines the need to provide a more appropriate niche. In addition, common transplantation sites such as liver and subcutaneous adipose tissue have significantly lower blood perfusion on a per tissue weight basis than the native pancreas. To improve graft maturity pre-implantation and provide immediate blood supply and function post implantation, we designed a highly-perfused matrix-based scaffold that simulates the native beta-cell niche better than current encapsulation techniques.
Methods:
We developed a scalable technology to enable interposition of 15um thick semi-permeable biomimetic membranes between parallel hierarchical channels within extracellular matrix hydrogels (n=15 full-scale scaffolds) to support >500kIEQs (Fig 1). To verify that perfusion would enable sensing of a signal, bi-directional diffusion across our membrane, and convection of a product similar in size to insulin, we seeded HEK cells that secrete luciferase in response to IFN. Once scaffold function was confirmed, we differentiated hypo-immune induced pluripotent stem cells (Hypo1, Pancella, Toronto, Ontario) into beta-cells[1]. Two grafts (~15,000 stem-cell beta-cell clusters, n=1; ~80ug recombinant c-peptide, n=1) were attached extra-corporeally to the circulation of a healthy porcine recipient. Insulin output was quantified by ELISA (Mercodia).
Results:
Based on experience with in vivo beta cell maturation, we expected that in vitro scaffold maturation would require weeks of bioreactor culture. To test if our system would support extended beta cell culture, we first seeded small-scale grafts with primary rat islets and found that after 24 hours of perfusion culture, insulin was produced at a stimulation index >5 comparable to static controls. Likewise, HEK cell seeded human-scale grafts secreted luciferase in response to IFN (93,552 at D2; 4,137,350 at D7 (n=3, RLUs)) confirming signal-in and product-out. Grafts populated with hypo immune iPS beta-cells secreted insulin into the scaffold perfusate as long as 18 days. When connected to a porcine recipient (Fig 2), iPSC derived beta-cell grafts secreted human c-peptide into the systemic circulation at a rate of 1.4IU/hr or 78IU/hr when loaded with recombinant c-peptide.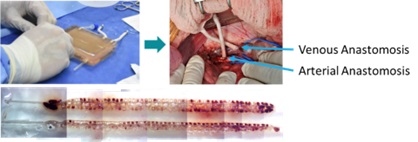
Conclusion:
The IVIVA graft design enables pre-implant maturation, glucose sensing and insulin secretion across a mechanical and endothelial based immune barrier, provides immediate blood supply at physiologic levels after implantation, and thereby supports beta-cell engraftment and long-term function. Anticipated next experiments include validation at scale with human islets and further large animal survival studies.
Juvenile Diabetes Research Foundation .
[1] Hogrebe, N.J., Maxwell, K.G., Augsornworawat, P., Millman, J.R. Generation of insulin-producing pancreatic β cells from multiple human stem cell lines. Nat Protoc. 16, 4109–4143 (2021). https://doi.org/10.1038/s41596-021-00560-y
Video Posters - Log in to view