
Restoring glucose homeostasis with stomach-derived human insulin-secreting organoids
Xiaofeng (Steve) Huang1, Wei Gu1, Ying Lan1, Jiaoyue Zhang1, Jonathan Colarusso1, Sanlan Li1, Qiao Zhou1.
1Medicine, Weill Cornell Medicine, New York, NY, United States
Introduction
Gut stem cells are accessible by biopsy and expand robustly in culture, providing abundant tissues for potential autologous transplantation therapies1-3. Insulin+ cells can be induced in mouse gut4, but it has not been possible to generate abundant and durable insulin-secreting cells from human gut tissues to evaluate their potential as a cell therapy for diabetes. We established a robust protocol to differentiate cultured human gastric stem cells (hGSCs) into pancreatic islet-like organoids containing gastric insulin-secreting (GINS) cells that resemble pancreatic β-cells and able to stably reverse diabetes after transplantation (doi.org/10.1101/2022.12.15.520488)5.
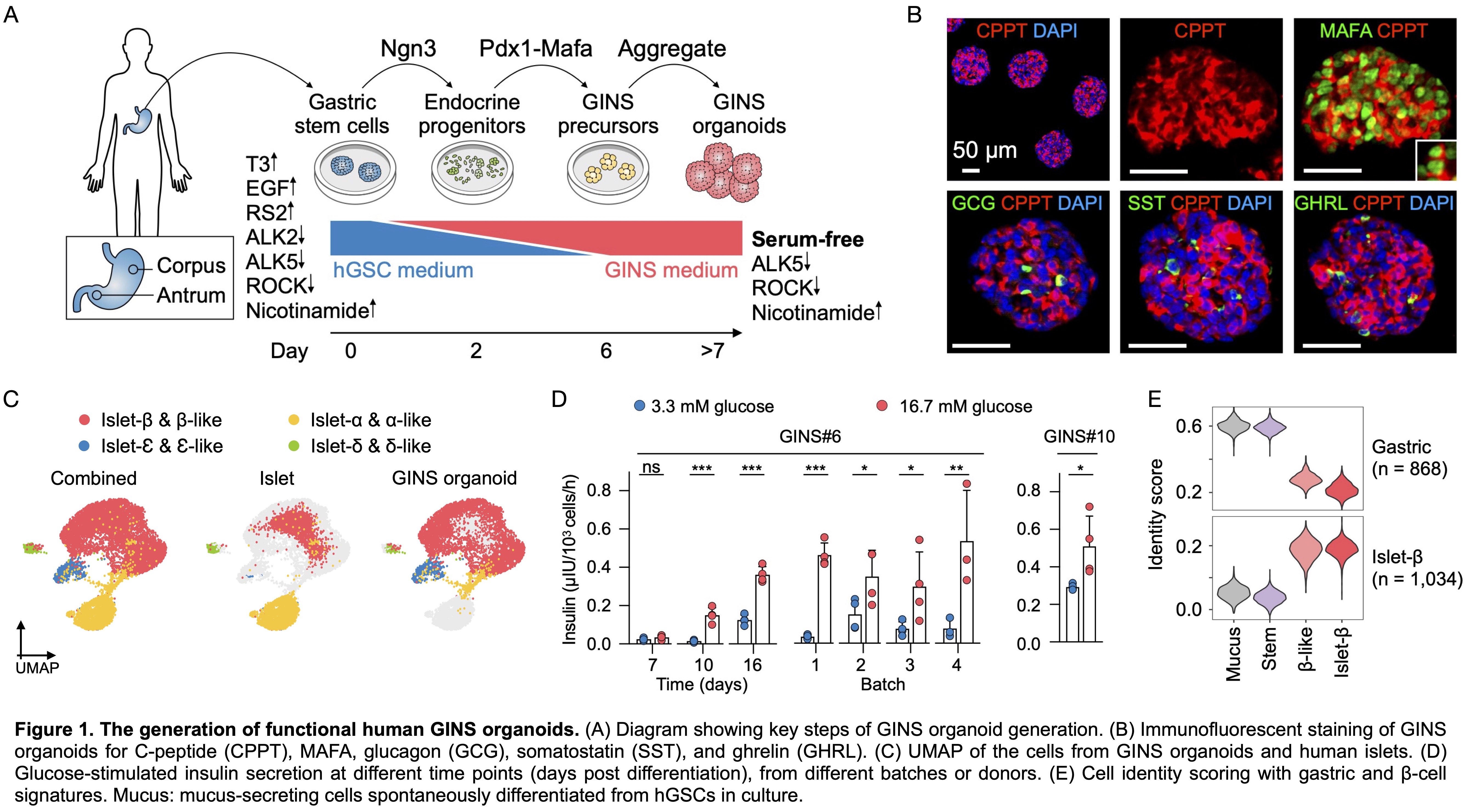
Method
Primary hGSCs derived from stomach biopsy or autopsy samples were expanded as colonies in culture. Sequential activation of the inducing factors NGN3 and PDX1-MAFA led to the formation of the GINS organoids (Fig. 1A). Functionality of GINS organoids were evaluated by statistic and dynamic glucose-stimulated insulin secretion (GSIS) assays. Transplantation study was conducted to evaluate their functionality in vivo including GSIS, glycemic control and glucose tolerance. scRNA-seq was performed to characterize the molecular profiles of GINS organoids before and after engraftment in comparison with human islets.
Results
Each biopsy-sized gastric sample typically yielded 30-40 primary hGSC colonies, which can be amplified to 109 cells within 2 months. After sequential activation of the inducing factors, hGSCs transitioned through a SOX4High endocrine and a GALHigh GINS precursor state before adopting the β-cell fate at efficiencies approaching 70% (Fig. 1B). scRNA-seq showed that GINS organoids contained four endocrine cell types that resembled islet β-, α-, δ-, and δ-cells (Fig. 1C). GINS organoids produced from multiple donors rapidly acquired glucose responsiveness, 10-12 days post differentiation (Fig. 1D). GINS cells expressed key genes involved in β-cell identity, metabolism, insulin secretion, and ion channel activities. To further assess cell identity, we applied molecular scorecards of β-cells (1,034 β-cell-specific genes) and gastric cells (868 stomach-specific genes) benchmarked from published human scRNA-seq data6,7. GINS cells scored high in β-cells and low in gastric signature, similar to islet β-cells (Fig. 1E). To characterize GINS cells in vivo, we transplanted GINS organoids under the kidney capsule of immunodeficient NSG mice. The grafts could live for more than 6 months and contained abundant insulin+ cells and exhibited GSIS (Fig. 2A, B). Transplantation of 6-8 million GINS cells into STZ-induced diabetic mice rapidly reversed hyperglycemia and maintained glucose homeostasis for over 100 days until graft removal (Fig. 2C). Glucose tolerance was improved significantly in the rescued mice (Fig. 2D).
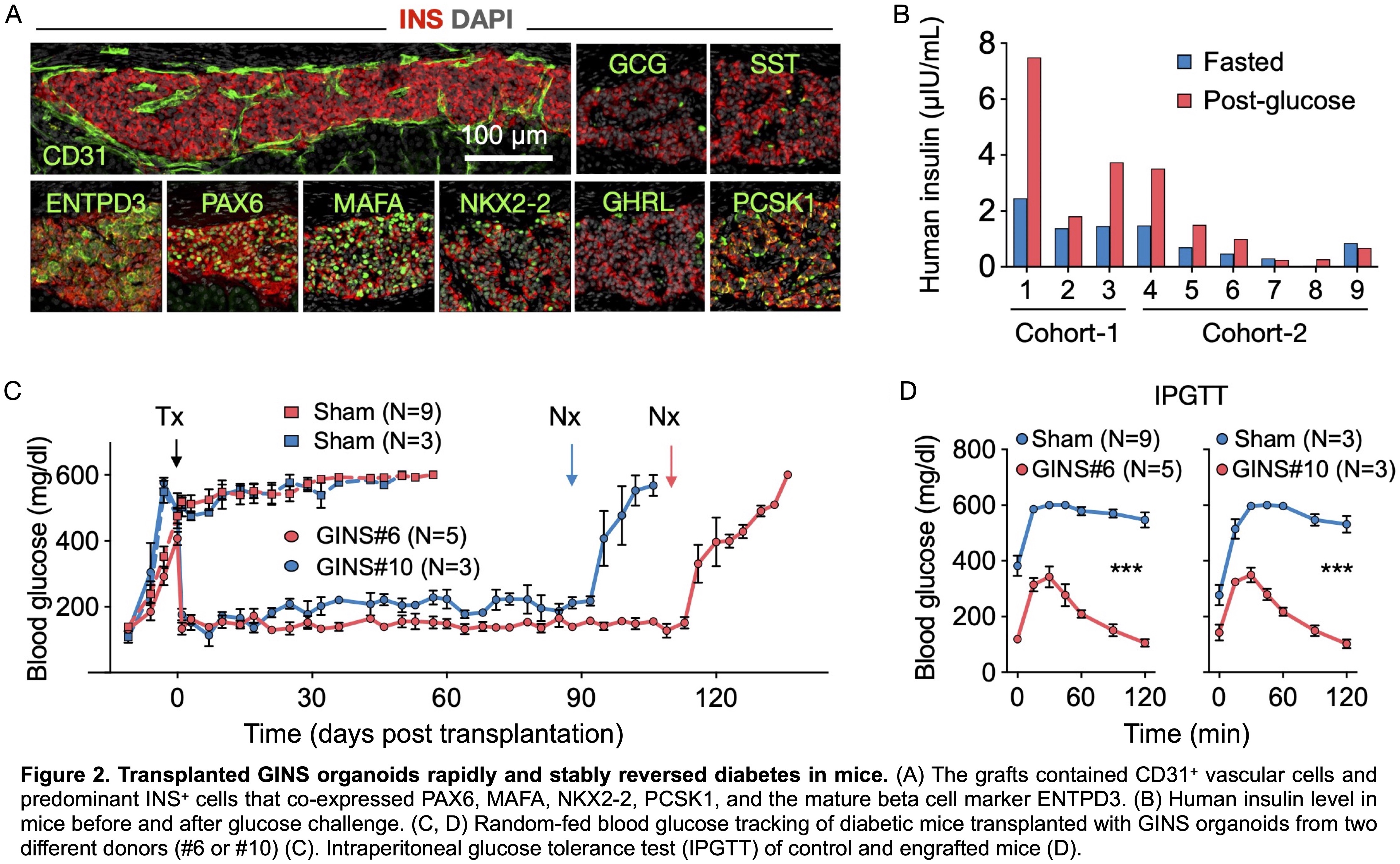
Conclusion
Our study established a promising approach to procuring autologous stomach-derived human insulin producers for diabetes treatment.
R01 DK106253. R01 DK13332. R01 DK125817. UC4DK116280.
[1] Sugimoto, S. et al. An organoid-based organ-repurposing approach to treat short bowel syndrome. Nature 592, 99–104, doi:10.1038/s41586-021-03247-2 (2021).
[2] Nikolaev, M. et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature 585, 574–578, doi:10.1038/s41586-020-2724-8 (2020).
[3] Meran, L. et al. Engineering transplantable jejunal mucosal grafts using patient-derived organoids from children with intestinal failure. Nat Med 26, 1593–1601, doi:10.1038/s4l59l-020-1024-z (2020).
[4] Egozi, A. et al. Insulin is expressed by enteroendocrine cells during human fetal development. Nat Med 27, 2104–2107, doi:10.1038/s41591-021-01586-1 (2021).
[5] X Huang, et al. Restoring glucose homeostasis with stomach-derived human insulin-secreting organoids. bioRxiv, 2022.12. 15.520488. doi: 10.1101/2022.12.15.520488 (2022).
[6] Muraro, M. J. et al. A Single-Cell Transcriptome Atlas of the Human Pancreas. Cell Sy st 3, 385–394 e383, doi:10.1016/j.cels.2016.09.002 (2016).
[7] Busslinger, G. A. et al. Human gastrointestinal epithelia of the esophagus, stomach, and duodenum resolved at single-cell resolution. Cell Rep 34, 108819, doi:10.1016/j.celrep.2021.108819 (2021).
Best Abstracts Session