Nutrient control of insulin secretion in stem cell-derived islets
Chencheng Wang1,2, Aleksandra Aizenshtadt2, Shadab Abadpour1,2, Andrea Dalmao Fernandez6, Merete Høyem1, Justyna Stokowiec2, Petter Angell Olsen2,5, Simona Chera3, Luiza Ghila3, Helge Ræder3,4, Stefan Krauss2,5, Hanne Scholz1,2.
1Department of Transplant Medicine and Institute for Surgical Research, Oslo University Hospital, Oslo, Norway; 2Hybrid Technology Hub, Center of Excellence, University of Oslo, Oslo, Norway; 3Department of Clinical Science, University of Bergen, Bergen, Norway; 4Department of Pediatrics, Haukeland University Hospital, Bergen, Norway; 5Department of Immunology and Transfusion Medicine, Oslo University Hospital, Oslo, Norway; 6Section for Pharmacology and Pharmaceutical Biosciences, Department of Pharmacy, niversity of Oslo, Oslo, Norway
Introduction: Stem cell-derived islets (SC-islets) are unlimited human beta cell sources for transplantation that could provide a curative diabetes treatment. However, few in vitro generated SC-islets achieve an in vitro glucose-stimulated insulin secretion (GSIS) response equivalent to primary human islets in terms of the magnitude of insulin secretion or a biphasic pattern of insulin release1.
Methods: We here optimized the SC-islets differentiation protocol by an additional WNT signaling pathway inhibition at stage 2. The differentiation efficiency was evaluated by flow cytometry analysis. Dynamic and static insulin secretion of the SC-islets derived from the optimized protocol in response to different nutrient sources, including glucose, pyruvate, amino acid, and palmitate, were investigated. The oxygen consumption rate (OCR) of SC-islets in response to different nutrient sources was quantified by the Seahorse analysis. Finally, SC-islets derived from the optimized protocol were transplanted in mice under kidney capsule to examine the in vivo functionality.
Results: Our data showed that the WNT inhibition at stage 2 significantly increased Chromogranin A positive endocrine cell (P<0.001) and C-peptide positive cell (P<0.001) population in the studied induced pluripotency stem cell line than non-WNT inhibition at stage 2 (Figures A and B). SC-islets can maintain GSIS ability for 4 weeks in vitro with an average secretion index (SI) of 1.45. The average SI significantly decreased in week 6. However, this prolonged in vitro culture decreased glucagon-insulin and somatostatin-insulin bi-hormone cell fraction (Figures C and D). SC-islets from 3 batches of differentiation showed an elevated OCR in response to 20 mM glucose stimulation (Figure E). Dynamic GSIS shows that SC-islets respond to pyruvate and glucose stimulation (Figure F). Glutamine cannot induce insulin secretion in human islets and SC-islets; leucine induces elevated insulin secretion in human islets but not in SC-islets. Furthermore, the combination of 20 mM glucose and amino acid amplifies insulin secretion in human islets but not in SC-islets (Figures G and H). Palmitate alone cannot induce insulin secretion in human islets and SC-islets. When glucose presences, palmitate amplifies insulin secretion in human islets, not in SC-islets (Figures I and J). Transplantation of 600 SC-islets under the kidney capsule successfully prevented alloxan-induced diabetes in 3 out of 3 mice.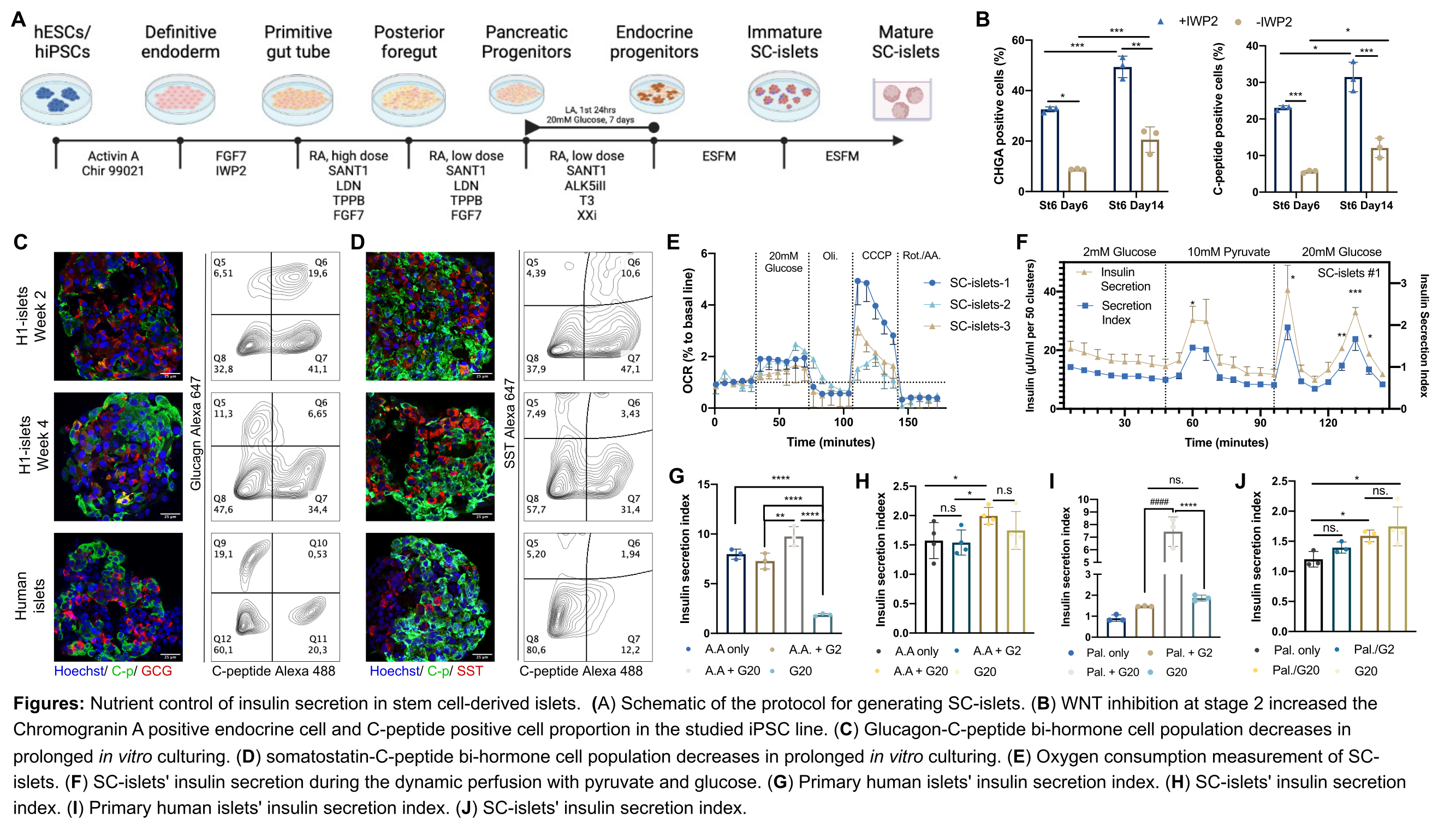
Conclusions: The SC-islets differentiation efficiency in this study was ensured through an optimized planar differentiation protocol. SC-islets’ insulin secretion in response to different nutrient sources was different from human islets, although they showed elevated OCR in response to glucose stimulation. This study provides a reference for further optimizing SC-islets differentiation protocol by targeting the different nutrient metabolism processes.
We thank Oslo University Hospital core facilities for the use of microscopy and flow cytometry. This work has been supported by UiO: Life Science, the Research Council of Norway through its Centers of Excellence funding scheme, project number 262613, and the Norwegian Diabetes Association..
[1] Davis, J. C. et al. Glucose Response by Stem Cell-Derived β Cells In Vitro Is Inhibited by a Bottleneck in Glycolysis. Cell Rep. 31, 107623 (2020).
Best Abstracts Session