
Harald Stover, PhD
Founder and CEO, Allarta Life Science Inc.
Over his 30 years as a Professor at McMaster University, Dr. Harald Stover has become a leading academic researcher in polymer hydrogels, bio-relevant macromolecules and the delivery of biologics. His work is published in leading academic journals, and he is a frequent speaker at international scientific conferences.
His awards include an NSERC/3M Industrial Research Chair, Canada’s National Award for Macromolecular Science and Engineering, and his appointment as Director of the NSERC Collaborative Research and Training Experience (CREATE) Program in Biomaterials.
Harald earned his BSc from Technische Universität Darmstadt in Germany, his PhD from the University of Ottawa, and completed his post-doctoral work at Cornell University.
Harald is co-founder and CEO of Allarta Life Science Inc., a company focussed on delivering cell-based therapies, based on its unique hydrogel platform technology.
Immuno-privileged hydrogels protect functionality of islets transplanted into immunocompetent diabetic animals without immunosuppression
Harald Stover1.
1Allarta Life Science Inc., Hamilton, ON, Canada
Introduction: Despite the promise of islet cell transplantation for people with T1D, the need for systemic immune suppression, and its associated side effects, still presents a serious hurdle. Until safe and immune-evasive cells are validated, physical immuno-protection can help enable allogeneic islet implants. Our proprietary crosslinked, synthetic hydrogels provide shape-agnostic, robust, and retrievable Islet environments with non-immunogenic properties.
Methods: The hydrogels were assessed for in vitro mechanical robustness, using micropipette aspiration, chemical stress tests (e.g., citrate), and in vivo persistence. Protein release profiles and diffusion limits were evaluated, using FITC-labeled dextran (10, 70, 250, and 500 kDa), IgG (150 kDa), and insulin (5.8 kDa). A variety of modifications were tested for toxicity towards human induced pluripotent stem cells (hiPSCs) and in vivo responses in healthy mice and pigs. Furthermore, encapsulated rat and human donor islets were assessed for in vitro functionality (e.g., GSIS) and in vivo responses by implanting marginal islet loads in the peritoneal space of streptozotocin (STZ)-induced diabetic immunocompetent mice. In vivo islet functionality was assessed over time through measurement of blood glucose, C-peptide, and HbA1c. In-vitro and in-vivo functionality of next-generation shape-agnostic hydrogels were also assessed.
Results: Our next-gen, shape-agnostic hydrogels proved to be both robust, and effective in excluding FITC-labeled IgG and larger molecules, while allowing rapid out-diffusion of insulin. The hydrogels maintained good islet function in vitro, shown by high GSIS indices. We assessed several novel immune-evasive modifications and found favorable impact on cell metabolic activity in vitro. Capsules were retrieved intact after up to ~300 days in vivo. Encapsulated islets enabled rapid and persistent blood glucose control for 140+ days in immunocompetent STZ-diabetic C57BL/6 mice using a marginal load of rat islets. Human donor islet function in mice was extended to day 45 (~8x longer than reported for free human islets), with C-peptide, appropriate HbA1c decreases, and minimal foreign body response.
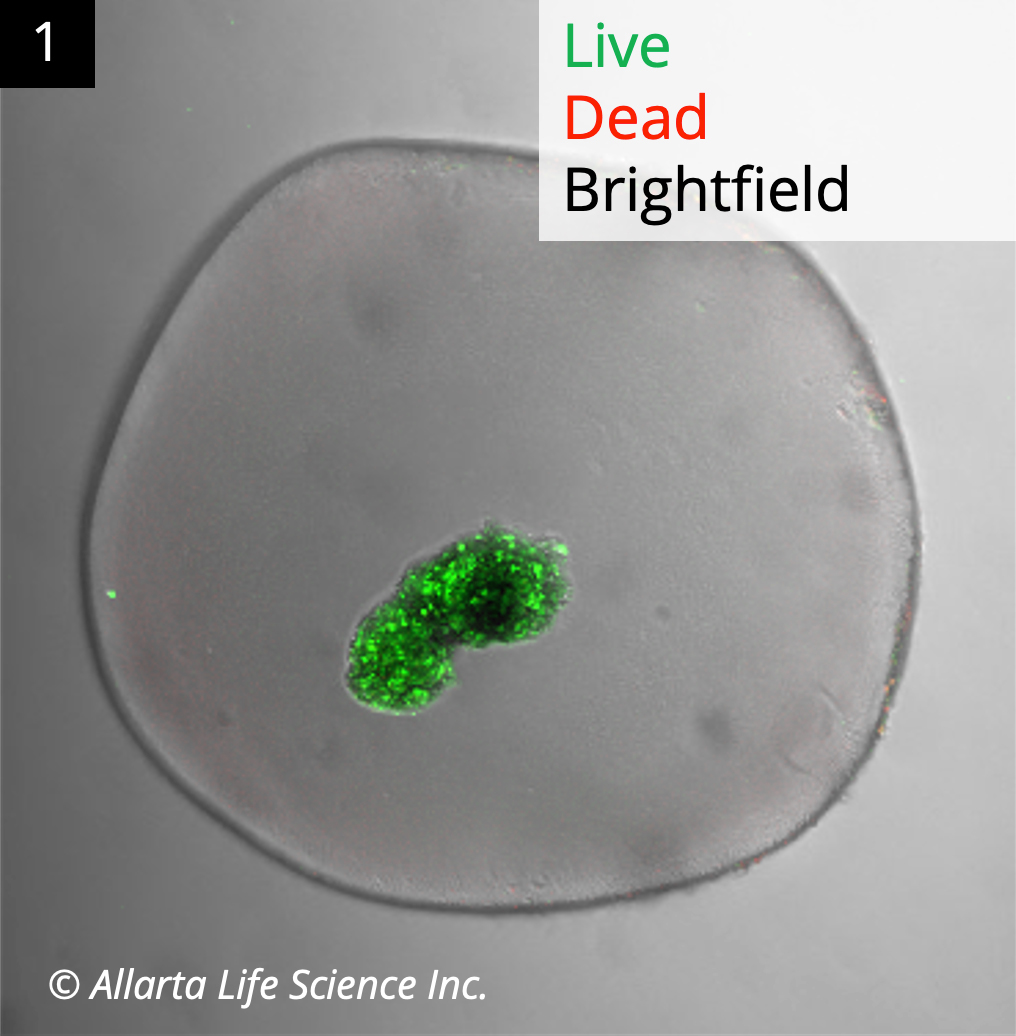 Clear capsules with viable human donor islets were explanted from normal mixed breed pigs after three weeks (Image 1). Detectable systemic human C-peptide levels from marginal human Islet transplants demonstrated the functionality of donor islets in a large animal model.
Clear capsules with viable human donor islets were explanted from normal mixed breed pigs after three weeks (Image 1). Detectable systemic human C-peptide levels from marginal human Islet transplants demonstrated the functionality of donor islets in a large animal model.
The technology supports arbitrary hydrogel shapes with centered placement of Islets (Figure 2, Live/Dead-stained Islets in gel string). 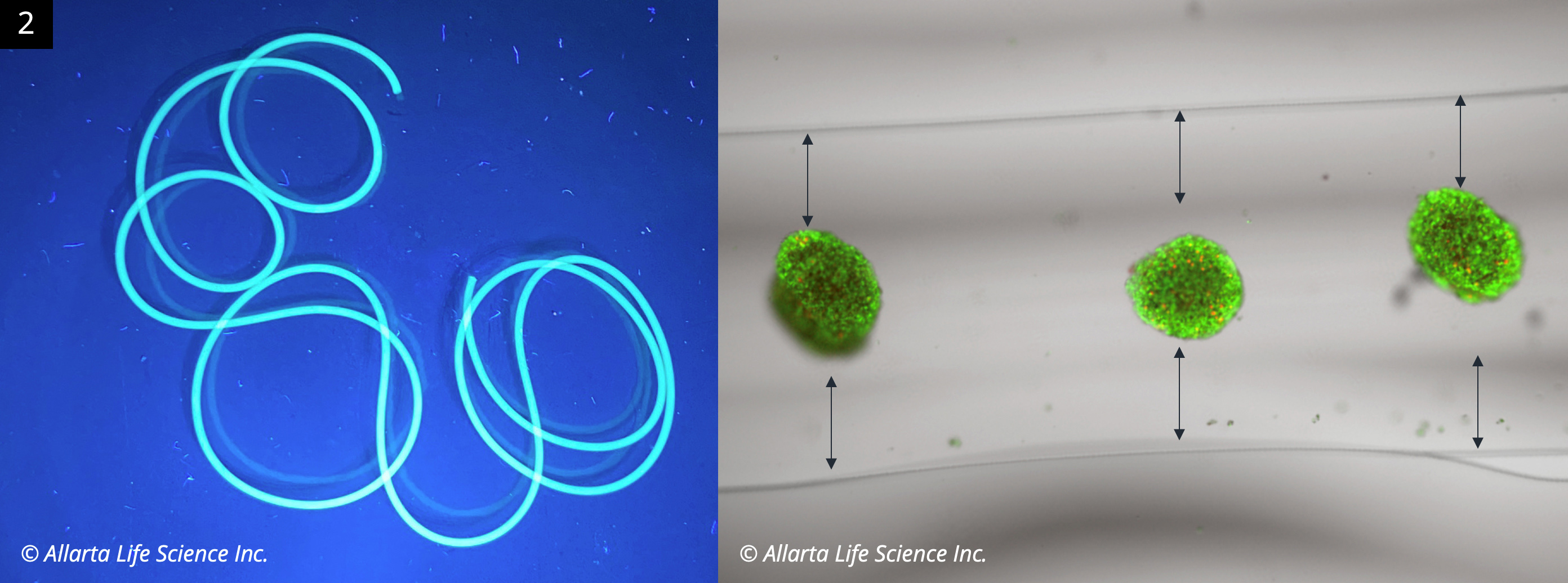
Conclusion: Our proprietary synthetic and retrievable hydrogels show immune protection in xenogenic transplants of donor islets into immune-competent animals. We are currently scaling to therapeutic doses in large animals in pursuit of a curative human allogeneic therapy for T1D based on an optimal balance of Islet protection and functionality without immunosuppression.
Best Abstracts Session