
Pre-vascularized bioartificial pancreas device development using 3D printing
Corinne Hoesli1,2, Jonathan A Brassard2, Brenden N Moeun1, Yannick Rioux3, Marco Gasparrini4, Richard L Leask1,2, Steven Paraskevas4,5, Timothy J Kieffer6, André Bégin-Drolet3, Jean Ruel3, Sophie Lerouge7.
1Chemical Engineering, McGill University, Montreal, QC, Canada; 2Biomedical Engineering, McGill University, Montreal, QC, Canada; 3Mechanical Engineering, Université Laval, Québec, QC, Canada; 4Surgery, McGill University Health Center, Montreal, QC, Canada; 5Surgery, McGill University, Montreal, QC, Canada; 6Cellular and Physiological Sciences, University of British Columbia, Vancouver, BC, Canada; 7Mechanical Engineering, École de technologie supérieure (ÉTS), Montreal, QC, Canada
With improvements in stem cell-derived islet (SC-islet) biomanufacturing protocols and ongoing clinical studies, the need to better understand the limitations and improve the design of cell delivery systems has become clear. Our teams are developing various pre-vascularized devices or engineered tissues either to study the impact of vascular networks on SC‑derived islets in vitro or to improve SC-islet survival and function in vivo (Figure 1).
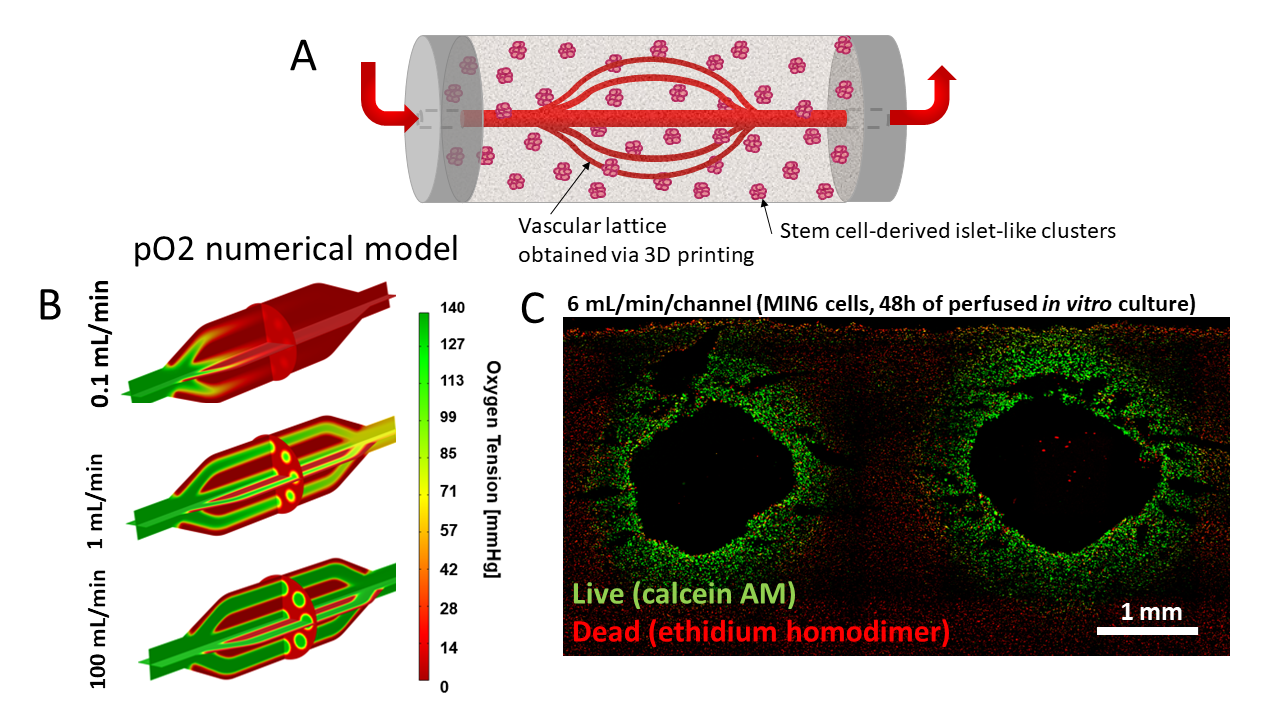
In one project, we are developing a vascular lattice device that would anastomosed to existing vasculature. The current device is obtained through 3D printing of vascular lattice templates using carbohydrate glass as sacrificial material. The carbohydrate glass lattices are then coated with polyurethane via solvent casting and particulate leaching to obtain a porous membrane after dissolution of the lattice template. An external compartment is obtained in a similar manner. These devices show excellent mechanical properties for use as vascular grafts, including high suture retention strength and burst pressures. The external compartment can be filled with a mixture of SC-islets and an immunoprotective formulation of alginate to combine the advantage of vascularization and immunoprotection. MIN6 clusters immobilized in this system show glucose-responsive insulin secretion under in vitro perfusion. SC-islets can be maintained viable for a week of in vitro culture. We are investigating physiological responses of SC-islets in vitro and adapting device design for implantation into small and large animals.
Another approach is to 3D print sacrificial materials directly into a cell-laden hydrogel support matrix using embedded writing. Compared to more conventional extrusion-based techniques, this approach reduces the printing time. It also enables implementation of a broader range of materials to support 3D culture of SC-islets.
The 3D printing approach leads to versatile platforms in terms of geometry and implementation with a variety of cell types and hydrogels. We envision the application of these strategies to generate models to study the combinatorial effects of oxygenation, endothelial interactions with SC-islets and molecules that can be perfused through the tissue constructs. These vascularized bioartificial tissues can be implemented to study fundamental SC-islet but also developing or adult pancreas biology. The polyurethane devices are also particularly adapted for transplantation applications.
This research was undertaken thanks to funding from JDRF (1-PNF-2016-249-S-B & 5-SRA-2021-1150-S-B), Diabetes Québec (Subvention pour la recherche 2014), the Quebec Cell, Tissue and Gene Therapy Network –ThéCell (a thematic network supported by the Fonds de recherche du Québec–Santé) (Subvention de projets structurants 2021), the Natural Sciences and Engineering Research Council of Canada (C.H.: RGPIN-2020-05877, S.L.: RGPIN-2020-06684), the Canadian Insitutes of Health Research (CIHR, DT1-179094) and the Fonds de recherche du Québec - Nature et technologies (2022-PR-297722). Several other research networks also supported our teams, notably the Cardiometabolic Health, Diabetes and Obesity (CMDO) Research Network, the Quebec Centre for Advanced Materials (QCAM), the Montreal Diabetes Research Center (MDRC), and the PROTEO Network..
Best Abstracts Session